Pre-Seed® is referenced in the physicians' expert consensus report “Optimizing Natural Fertility”, a best practice guideline by the American Society for Reproductive Medicine.

Pre~Seed®’s unique, isotonic formula is covered by numerous issued US and international patents. As an OTC Class II Medical Device, it has the same quality requirements and lot release testing as for assisted reproduction media. Pre-Seed can be applied intravaginally prior to intercourse, to coat the vagina and exterior cervix with moisture balanced to match fertile cervical mucus in osmolality, pH and viscosity.
Dr. Ellington, the inventor of Pre~Seed®, has received funding through the NIH for over twenty years, and has an internationally recognized curriculum vitae of publications in sperm physiology.
Pre-Seed’s isotonic formula is also available in an external lubricant, for everyday patient or clinic use, as Pre’® Lubricant. Both Pre~Seed and Pre´have been used for lubrication by women in all stages of life, including post-menopausal women and women with pelvic pain disorders, such as vulvodynia. Read what other Physicians and Medical Staff are saying about our uniquely mild products!
INGfertility Quality Assurance
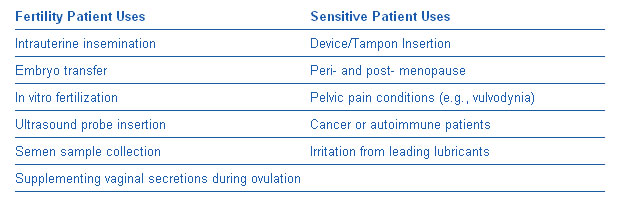
INGfertility’s lubricants are the first and currently only products in the United States that are allowed to claim that they:
- are safe for use by couples who are trying to conceive;
- don’t harm sperm or embryos; and
- are appropriate for use during fertility interventions, such as:
- - intrauterine insemination
- - in vitro fertilization
- - embryo transfer
- - transvaginal oocyte collection
Each lot of both Pre~Seed & Pre’ is tested to ensure no deleterious effect on human sperm or mouse embryos. This is a unique feature of our products. Osmolality, pH, viscosity and endotoxin are also all tested prior to the release of either product.
A detailed summary of all biocompatibility studies done on our products is available (info@ingfertility.com). These data have been reviewed and accepted by regulatory agencies throughout the world. Most of them have been done by third party laboratories.
Please contact us at info@ingfertility.com for a Certificate of Analysis on any Lot of product, or for more information on regulatory reviews or clinical studies.

Over the past decade, there has been a great deal of data generated by scientists in the USA and overseas confirming the safety of Pre~Seed’s formula and supporting its use in fertility medicine. These include data presented as peer-reviewed full publications and/or as presentations at major medical meetings.
These studies have been part of Pre~Seed’s review by agencies around the world and have lead to its designation as the first 'fertility-friendly' Class II Medical Device with labeling allowed of “safe to use when trying to conceive”.
Full Publications
Effect of Vaginal Lubricants on Sperm Motility and Chromatin Integrity: A Prospective Comparative Study - Fertility & Sterility. 2008 Feb;89:375-379
Agarwal A, Deepinder F, Cocuzza M, Short RA, Evenson DP. Reproductive Research Center, Glickman Urological Institute and Department of Obstetrics-Gynecology, Cleveland Clinic, Cleveland, Ohio
Objective: To evaluate the effect of vaginal lubricants Pre~Seed®, FemGlide®, Astroglide®, and Replens® on human sperm motility and chromatin integrity. Design: Prospective, comparative, in vitro study. Setting: Andrology laboratory at tertiary care hospital. Patient(s): Thirteen normozoospermic donors. Intervention(s): Semen samples from 13 subjects were incubated in human tubal fluid media (HTF) controls and 10% (vol/vol) of Pre~Seed®, FemGlide®, Astroglide®, and Replens® lubricants. After 30 minutes, progressive sperm motility was assessed by light microscopy. Semen samples of 12 patients were placed in positive control (HTF), negative control (10% K-Y Jelly® lubricant), and 10% vol/vol Pre~Seed® and FemGlide® lubricants. After 4 hours culture, spermatozoa were analyzed for percent DNA fragmentation index with use of the acridine orange-based sperm chromatin structure assay. Main Outcome Measure(s): Sperm motility and percent DNA fragmentation index. Results: Percent motility did not differ significantly between HTF controls and Pre~Seed®, whereas FemGlide®, Replens®, and Astroglide® lubricants demonstrated a significant decrease in motility. There was no significant difference in percent DNA fragmentation index between the HTF controls and Pre~Seed®, but a significant decline in sperm chromatin quality occurred with FemGlide® and K-Y Jelly®. Conclusion: Pre~Seed® does not cause a significant decrease in progressive sperm motility or chromatin integrity in contrast to other lubricants used by couples.

Mucosal Irritation Potential of Personal Lubricants Relates to Product Osmolality as Detected by the Slug Mucosal Irritation Assay - Sexually Transmitted Diseases. 2008 May;35:512-516
Els Adriaens, PhD; Jean Paul Remon, PharmD. Laboratory of Pharmaceutical Technology, Ghent University, Harelbekestraat 72, 9000 Ghent, Belgium
BACKGROUND: The slug mucosal irritation assay has recently been used as a sensitive measure of mucus membrane tolerance for vaginal microbicide products and carriers. In the current study, it was determined whether mucosal irritation potency of personal lubricants is related to varying product osmolalities.
METHODS: Five commercial lubricants with an osmolality range were evaluated using the previously validated slug mucosal irritation assay. Specifically, Arion lusitanicus were treated with lubricants over 5 days to quantify mucus production and tissue damage, allowing assignment of each product into an irritation potency category (none, mild, moderate, or severe). RESULTS: The irritation potency (assessed by the mucus production) of the lubricants showed a significant, quadratic relationship with the product osmolality (P = 0.001; R (2) = 0.99). Femglide, a hypo-osmotic lubricant (32 mOsm/kg), caused a negative mucus production. Pré, an iso-osmotic lubricant (316 mOsm/kg), caused no changes. Two moderately hyperosmotic lubricants, Replens and K-Y jelly (2143 and 2463 mOsm/kg), induced mild and moderate irritation, respectively. The highly hyperosmotic lubricant Astroglide (5848 mOsm/kg) resulted in severe irritation and tissue damage. CONCLUSIONS: Commonly used personal lubricants show a full range of mucosal irritation potential, which is related to product osmolality.

Sperm Toxicity of ‘Nonspermicidal’ Lubricant and Ultrasound Gels Used in Reproductive Medicine - In preparation for submission 2010
Josefina Vargas, M.Sc.; Michel Crausaz, M.Sc.; Alfred Senn, Ph.D.; Marc Germond, M.D. Fondation F.A.B.E.R., Rue de la Vigie 5, 1003 Lausanne, Switzerland
Objective: To compare sperm toxicity of four commercial “non-spermicidal” gels used in Reproductive Medicine including: Aquasonic Ultrasound Gel, Felis Lubricant, Pre-Seed Lubricant and Replens Moisturizer, in a range of concentrations (0.083% - 8.3%) believed to be physiologically relevant. Results: Sperm toxicity was observed as shown by significant declines in motility over the 24-hr assay following incubation with Aquasonic, Felis and Replens at higher gel concentrations. Although Replens, even at only 0.83% v/v resulted in sperm toxicity. Pre-Seed alone was not toxic to sperm at any concentration, including the highest (8.3%). Conclusions: Three of the “nonspermicidal” gels were toxic to sperm, including Aquasonic Gel which is widely used for transvaginal ultrasound during ovulation. Pre-Seed alone did not cause sperm toxicity at any time or concentration. It is appropriate for use by patients trying to conceive or clinicians during fertility procedures, including facilitating vaginal insertion of ultrasound probes.

Optimizing Natural Fertility - Fertility & Sterility. 2008 Nov;90(Suppl 3):S1-S6
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinolgy and Infertility
Synopsis: Some vagin al lubricants may decrease fertility, based on their observed effects on sperm survival in vitro. Whereas commercially available water-based lubricants (e.g., Astroglide®, KY Jelly®, and Touch®) inhibit sperm motility in vitro by 60% to 100% within 60 minutes of incubation, canola oil has no similar detrimental effect (32). KY Jelly, olive oil, and saliva diluted to concentrations even as low as 6.25% adversely affect sperm motility and velocity.... Hydroxyethylcellulose-based lubricants such as Pre-Seed® (INGfertility, Valleyford, WA) also have no demonstrable adverse impact on semen parameters (35). Although there is no evidence to indicate that use of any vaginal lubricant decreases fertility, it seems prudent to recommend... hydroxyethylcellulose-based lubricants when they are needed.

Dyspareunia and Vaginal Dryness after Breast Cancer Treatment - SRM Sexuality, Reproduction & Menopause. 2008 Aug;6:18-22
Doreen Leyden Wiggins, MD1 & Don S. Dizon, MD2. 1Clinical Assistant Professor, Program in Women's Oncology and 2Assistant Professor, Obstetrics and Gynecology and Medicine. Co-Directors, Center for Sexuality, Intimacy & Fertility, Women and Infants Hospital of Rhode Island, Providence, RI
An animal model using slugs has been validated to test mucosal irritation in human mucosal membranes. A recent study using the slug mucosal irritation assay determined that product osmolality was an important component to mucosal tolerability. The iso-osmotic lubricant (Pre-Seed) caused no changes and, therefore, was the best tolerated. The hypoosmotic lubricant (Femglide) caused negative mllCllS production, thereby decreasing natural response. Two moderately hyperosmotic lubricants (Replens, K-Y jelly) induced mild and moderate irritation, respectively. The highly hyperosmotic lubricant (Astroglide) resulted in severe irritation and tissue damage.Pre-Seed has been marketed to couples who need lubrication that does not inhibit fertility.
